This is the first tri-omic taxonomy of the human hippocampus, addressing two critical challenges in spatial biology. First, the missing cell morphology and extracellular matrix information were profiled by 25-plex sequential protein imaging (known as CODEX). This innovation also allowed us to create spatial maps of cell identity with single-cell resolution. Second, employing our in-house spatial mRNA and ATAC-seq platforms (DBiT-seq), we identified the RNA dynamics in different hippocampal subfields and open chromatin regions for gene regulation. Together, these efforts produced the most comprehensive spatial atlases of 16 human hippocampi, comprising over 1.6 million single cells.
Spatial Multi-Omic Taxonomy of Human Hippocampus
Spatial Technology Innovation
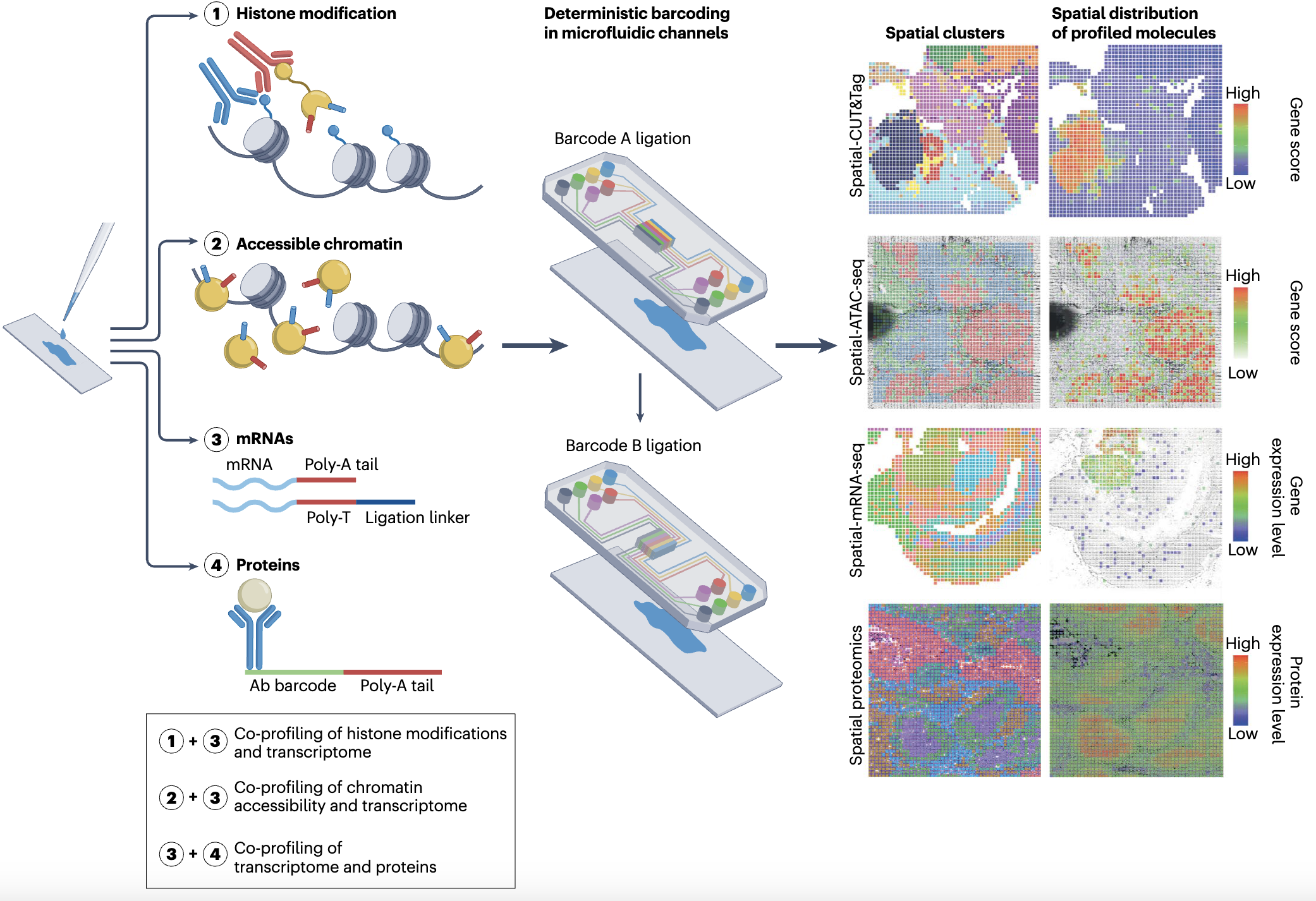
Spatial Technologies Based on Microfluidics
Image Credit: Deng et al., Nature Reviews Bioengineering, 2023.
-
Deterministic barcoding in tissue for sequencing (DBiT-seq) is a technique that combines spatial in situ barcoding with next-generation sequencing to map gene expression patterns across tissue sections. This innovation allows for high-resolution (up to 10 microns) profiling of mRNA within intact tissues, providing valuable insights into cellular organization and tissue heterogeneity.
Liu Y, Yang M, Deng Y, Su G, Enniful A, Guo C, Tebaldi T, Zhang D, Kim D, Bai Z, Norris E, Pan A, Li J, Xiao Y, Halene S, Fan R. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell, 183(6):1665-1681.e18 (2020).
-
Spatial ATAC-seq (Assay for Transposase-Accessible Chromatin with sequencing) enables the mapping of open chromatin regions in situ. By identifying regions of accessible chromatin, this technique provides information on the regulatory landscape of cell-type-specific gene expression potential across spatially defined areas in tissues.
Deng Y, Bartosovic M, Ma S, Zhang D, Kukanja P, Xiao Y, Su G, Liu Y, Qin X, Rosoklija GB, Dwork AJ, Mann JJ, Xu ML, Halene S, Craft JE, Leong KW, Boldrini M, Castelo-Branco G, Fan R. Spatial profiling of chromatin accessibility in mouse and human tissues. Nature, 609(7926): 375-383 (2022).
-
This is a groundbreaking approach for spatially resolved epigenome-transcriptome co-profiling in mammalian tissues, which enables simultaneous mapping of chromatin accessibility and gene expression at high resolution.
Zhang D*, Deng Y*, Kukanja P, Kukanja P, Agirre E, Bartosovic M, Dong M, Ma C, Ma S, Su G, Bao S, Liu Y, Xiao Y, Ma S, Rosoklija GB, Dwork AJ, Mann JJ, Leong KW, Boldrini M, Wang L, Haeussler M, Raphael BJ, Kluger Y, Castelo-Branco G, Fan R. Spatial epigenome–transcriptome co-profiling of mammalian tissues. Nature, 616(7955): 113-122 (2023).
-
This novel spatial technology allows for mapping non-coding RNAs (LncRNA, microRNA, tRNA etc) and coding RNA (mRNA) at the same time. It used Poly(A)Polymerase to conjugated a short sequence to all RNAs, thus allowing high capture efficiency of degraded RNAs in FFPE samples.
Bai Z*, Zhang D*, Gao Y*, Tao B*, Zhang D*, Bao S*, Enninful A, Wang Y, Li H, Su G, Tian X, Zhang N, Xiao Y, Liu Y, Gerstein M, Li M#, Xing Y#, Lu J#, Xu ML#, Fan R#. Spatially Exploring RNA Biology in Archival Formalin-Fixed Paraffin-Embedded Tissue. Cell, 187(23): 6760-6779.e24 (2024).
Brain Tumor Metastasis in the Perivascular Niche
Brain tumor cells hijacking the blood vessels as highway
Image Credit: Xiao et al., Advanced Science, 2019.
Dr. Xiao conducted her doctoral research on brain tumor invasion in the perivascular niche. Employing advanced techniques such as organ-on-a-chip and single-cell sequencing, her work explained why some primary glioblastoma cells prefer to reside close to vessels while others choose to stay and proliferate in the tumor mass.
Tumor cell growth and migration in the perivascular niche
Xiao Y, Kim D, Dura B, Zhang K, Yan R, Li H, Han E, Ip J, Zou P, Liu J, Chen AT, Vortmeyer AO, Zhou J, Fan R. Ex vivo dynamics of human glioblastoma cells in a microvasculature-on-a-chip system correlates with tumor heterogeneity and subtypes. Advanced Science, 6(8):1801531 (2019).Cell lineage trajectory of proliferative tumor versus invasive tumor
Chen AT*, Xiao Y*, Tang X*, Baqri M, Gao X, Reschke M, Zhou Y, Deng G, Zhang S, Deng Y, Bai Z, Kim D, Huttner A, Kunes R, Saltzman WM, Fan R, Zhou J. Cross-platform analysis reveals cellular and molecular landscape of glioblastoma invasion. Neuro-Oncology, 25(3):482-494 (2023).Building perfusable in vitro vascular network
Xiao Y, Liu C, Chen Z, Blatchley M, Gerecht S, Fan R. Senescent fibroblasts promote microvasculature formation in vitro. Advanced Biosystems, 3(8):1900089 (2019).
Single-Cell and Spatial Biology in Translational Clinical Research
-

Tendon Enthesis
This study reveals the cellular landscape in the tendon enthesis, the tissue interface where tendons attach to bones. We established a framework for understanding enthesis maturation and identified a potent Gli1-lineage progenitor with clonogenicity and multipotency that improves enthesis healing in an adult injury model.
Fang F, Xiao Y, Zelzer E, Leong KW, Thomopoulos S. A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell, 29(12):1669-1684 (2022).
-

Obesity
This study highlights a strategy to target visceral fat and suggests that cationic nanomaterials could be exploited for treating metabolic diseases. Single-cell RNA sequencing revealed that the polycations can inhibit the storage of lipids in fat cells, reducing the abdominal fat in obese mice.
Wan Q*, Huang B*, Li T, Xiao Y, He Y, Du W, Wang BZ, Dakin GF, Rosenbaum M, Goncalves MD, Chen S, Leong KW, Qiang L. Selective targeting of visceral adiposity by polycation nanomedicine. Nature Nanotechnology, 17(12):1311-1321 (2022).
Yu L, Yang YX, Gong Z, Wan Q, Du Y, Zhou Q, Xiao Y, Zahr T, Wang Z, Yu Z, Yang K, Geng J, Fried SK, Li J, Haeusler RA, Leong KW, Bai L, Wu Y, Sun L, Wang P, Zhu BT, Qiang L, Wang L. FcRn-Dependent IgG Accumulation in Adipose Tissue Unmasks Obesity Pathophysiology. (2024). (Cell Metabolism, In Press)
-

Substance Use Disorder
Understanding the impact of long-term opioid exposure on the embryonic brain is critical due to the surging number of pregnant mothers with opioid dependency. A human midbrain model is established that uses hiPSC-derived midbrain organoids to assess cell-type-specific responses to acute and chronic fentanyl treatment and fentanyl withdrawal. Single-cell mRNA sequencing reveals that chronic fentanyl treatment arrests neuronal subtype specification during early midbrain development and alters synaptic activity and neuron projection.
Kim HS*, Xiao Y*, Chen X, He S, Im J, Willner MJ, Finlayson MO, Xu C, Zhu H, Choi SJ, Mosharov EV, Kim HW, Xu B, Leong KW. Chronic opioid treatment arrests neurodevelopment and alters synaptic activity in human midbrain organoids. Advanced Science, 11(21): 2400847 (2024).
Study Cell Fates and Cell States by Spatial Omics
Cellular function and homeostasis are highly context-dependent; many critical biological processes arise when spatially organized factors like blood supply are disrupted, tissue barriers breach, or resource demands exceed availability. These spatially and temporally restricted events are central to pathologies such as injury, inflammation, and aging, where localized cells meets altered or compromised blood flow. The expression of each individual gene or cell may not be super accurate, but collectively, the cumulative information across many genes or cells is ample to decode a canonical function.
Key questions driving our research include:
What is a cell type? What is a cell state?
How do complex structures like the brain arise?
How do cells organize or maintain the microenvironment?
How do spatially altered metabolic conditions, such as ischemia, impact these cell-cell communication networks?
Are RNAs the best proxy for protein? How to understand their relationships?
How does transcription factors regulate the cell behavior in the brain?
How does the brain encode and store memory?
What determines the life span?